What Happens to Water Molecules During the Boiling Process
The molecules are now in the gaseous state. Two molecules of potassium and one molecule of sulfate.
12 4 Evaporation And Condensation Chemistry Libretexts
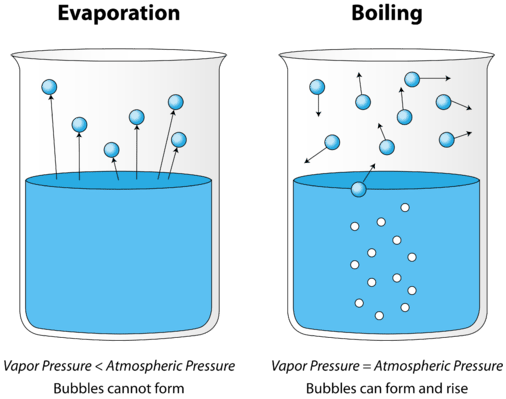
At a point the vapor pressure of the liquid becomes equivalent to the vapor pressure of the air around it.

. What happens to water molecules during the boiling process. Due to this absorption of energy the hydrogen bonds connecting water molecules to one another will break. This breaking of bonds between water molecules consumes any additional thermal energy added so that water.
When water boils the bonds holding the water molecules together as a liquid intermoleular forces are broken and the molecules are allowed to. Eventually the molecules have too much energy to stay connected as a liquid. When this occurs they form gaseous molecules of water vapor which float to the surface as bubbles and travel into the air.
The water vapor rises leaving less liquid water then there was before. A Boiling is a cooling process so energy is removed as quickly as it is added. The molecules move and vibrate so quickly that they escape into the atmosphere as molecules of water vapor.
Water molecules gain energy and increase the distance among its neighboring water molecules an increase in potential energy. The molecules of water dont break apart into hydrogen and oxygen. They move more slowly but move farther apart as they lose heat.
Eventually the molecules have too much energy to stay connected as a liquid. They move faster and move farther apart as they lose heat. When this occurs they form gaseous molecules of water vapor which float to the surface as bubbles and travel into the air.
What happens to water molecule when water boils. B Boiling is a cooling process so condensing steam inside bubbles cools the liquid as fast as it is heated. When water is boiled the heat energy is transferred to the molecules of water which begin to move more quickly.
I am goimg to explain this process based on the kinetic molecular theory. 8 What are liquid particles. When water is boiled the heat energy is transferred to the molecules of water which begin to move more quickly.
What happens to water molecules during the evaporation process. As you boil water the bonds between water molecules are broken and they turn into gas. Finally these molecules collect together to form a liquid.
7 What happens to particles during sublimation. They move slowly but move farther apart as they lose heat. The compound potassium sulfate K 2 SO 4 is composed of.
They move closer to other gas molecules. They move faster and move farther apart as they absorb heat. Evaporation happens when a liquid substance becomes a gas.
When water is boiled it undergoes a physical change not a chemical change. Boiling-hot water will evaporate quickly as steam. Once water evaporates it also helps form clouds.
Eventually the molecules have too much energy to stay connected as a liquid. 2 atoms of potassium 4 atoms of sulfur and 4 atoms of oxygen. Why doesnt energy added to boiling water increase the temperature of the water.
11 How are particles in liquid. What happens to water molecules during the boiling process. 2 atoms of potassium 1 atom of sulfur and 4 atoms of oxygen.
This theory says that everythingis made of small particles named molecules. They move faster and move farther apart as they lose heat. Melting is a process that occurs when matter changes its state from a solid to a liquid.
The water molecules begin to move faster with the increase in energy. What happens to water molecules during the boiling process. When this occurs they form gaseous molecules of water vapor which float to the surface as bubbles and travel into the air.
They move faster and farther apart as they absorb heat. Condensation happens when molecules in a gas cool down. Boiling water evaporates into thin air.
When water is heated it evaporates. When water is boiled the heat energy is transferred to the molecules of water which begin to move more quickly. This is the boiling point.
Eventually the molecules have too much energy to stay connected as a liquid. What change in energy happens when water droplets condense on the outside of a cup that holds a cool drink. They move faster and remain close together as they absorb heat.
The vapor pressure of the atmosphere changes at different altitudes which is why the boiling point changes. 12 What happens to particles when heat is constant. This is called water vapour.
10 Do particles move in a liquid. Eventually the molecules have too much energy to stay connected as a liquid. When this occurs they form gaseous molecules of water vapor which float to the surface as bubbles and travel into the air.
When water is boiled the heat energy is transferred to the molecules of water which begin to move more quickly. As the molecules lose heat they lose energy and slow down. 9 When a liquid is heated at its boiling point the.
In fact when a pure liquid boils the temperature of the liquid does not change therefore the average kinetic energy of the liquid does not change. Water boils when the thermal energy in the water which is a type of kinetic energy which causes the water molecules to move around exceeds the strength of the hydrogen bonds between the molecules causing them to separate from the other molecules. Instead the bonds between molecules of water break allowing them to change physically from a.
The water molecules in the water absorb that energy individually. 13 What is the particle structure of liquids. Evaporation is the opposite of condensation the process of water vapor turning into liquid water.
When water is boiled the heat energy is transferred to the molecules of water which begin to move more quickly. Answer 1 of 2. 6 What happens to particles during condensation.
They move faster and remain close together as they absorb heat. When this occurs they form gaseous molecules of water vapor which float to the surface as bubbles and travel into the air. The water level DECREASES because.

Why Boiling Actually Is A Cooling Process Your Brain On Sci
Q What S The Difference Between Evaporation And Boiling Nsta

Weird Science Macroscopic Changes In Liquid Water Volume Manoa Hawaii Edu Exploringourfluidearth
No comments for "What Happens to Water Molecules During the Boiling Process"
Post a Comment